electron configuration of fe|electron configuration periodic table : Tagatay How to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh . Sumakay ng Walang Pahintulot si Lotlot Kay Bobot . 04:28 HD. Babe Ang Haba Pala Ipasok Mo Nga
PH0 · full electron configuration cu
PH1 · fe2+ electron configuration condensed
PH2 · fe +2 def how many electrons added
PH3 · electron configuration periodic table
PH4 · electron configuration for every element
PH5 · electron configuration chart
PH6 · electron configuration calculator
PH7 · complete electron configuration
PH8 · Iba pa
Time Difference. Japan Standard Time is 16 hours ahead of Pacific Daylight Time 5:00 am 05:00 in JST is 1:00 pm 13:00 in PDT. JST to PST call time Best time for a conference call or a meeting is between 9:30pm-11:30pm in JST which corresponds to .
electron configuration of fe*******The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. How to Write .In order to write the Calcium electron configuration we first need to know the .Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .
How to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh .Potassium (K) - Electron Configuration for Iron (Fe, Fe2+, and Fe3+) - UMDIn order to write the Mg electron configuration we first need to know the .Chlorine (Cl) - Electron Configuration for Iron (Fe, Fe2+, and Fe3+) - UMDIn order to write the Sulfur electron configuration we first need to know the .Iron (Fe, Fe 2+, Fe 3+) Read my . Electron Configuration Notation:-shows the . Mar 23, 2023 Learn the electronic configuration of iron (Fe) with 26 electrons, its oxidation states, allotropic forms and applications. Find out how iron forms bonds, .
The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals. So, . Learn how to determine the ground state electron configuration of iron (Fe) using the Aufbau diagram and the periodic table. See the answer and explanation from two different sources.
Learn how to write the electronic configuration of iron (Fe) and its oxidation states (+2 and +3) with examples and diagrams. Also, find out the valency and applications of iron in . The electronic configuration of \[Fe^{2+} is - 1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 3d^{6},\] while that of \[Fe^{3+} is 1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 3d^{5}\]. \[Fe^{2+}\] .electron configuration periodic tableSo based on what we know about the quantum numbers and using the chart above, you need 2 electrons to fill an s orbital, 6 electrons to fill a p orbital, 10 electrons to fill a d orbital and 14 electrons to fill the f orbital.
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that . The ground state electron configuration of Fe is: "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"3d"^6"4s"^2" For all but about 20 transition metals, the Aufbau diagram is a useful tool that helps to determine the . In order to write the electron configuration for Iron (Fe) we first need to know the number of electrons for the Fe atom (there are 26 electrons). When we.Electron configurations of the elements (data page) This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the .
In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .electron configuration of fe electron configuration periodic tableOrbital diagram. Iron electron configuration. ← Electronic configurations of elements. Fe (Iron) is an element with position number 26 in the periodic table. Located in the IV period. Melting point: 1535 ℃. Density: 7.87 g/cm 3 . Electronic configuration of the Iron atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 .
Start with the electron configuration of the neutral atom. It is sufficient to use the noble gas configuration. Fe: [Ar] 4s 2 3d 6. Then, remove (or add) electrons to reflect the oxidation state of the metal. Remember to remove electrons from the orbital with the highest principal quantum number first! Fe(III) has lost 3 electrons relative to .Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. . What is the electronic configuration of #Fe^(3+)# ion? Question #19968. Question #3c8ff. What is the electronic configuration of magnesium? Question #3df7e. Question #32589. To know the abbreviated fe3+ electron configuration first of all we must know the abbreviated electronic configuration of fe atom. The abbreviated fe electron configuration is [Ar] 3d6 4s2. By counting the electrons from 1s to 3p orbital we find that there is a total number of 18 electrons and we replace it by writing in the form of [Ar]. The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).
The Electron: Crash Course Chemistry #5. Video 2.6.2 2.6. 2: An overview of the role of orbitals in electron configurations and how to write electron configurations. The relative energy of the subshells determine the order in which atomic orbitals are filled (1 s, 2 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, and so on).
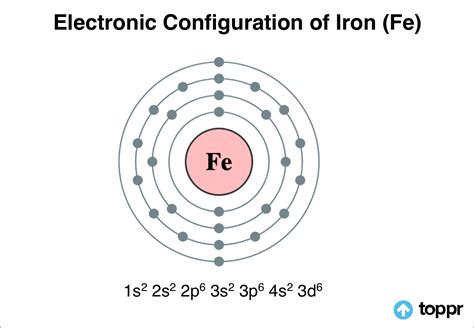
The number of electrons in the valence shell of iron (Fe) can be used to calculate the electronic configuration of the various oxidation states. Fe2+ F e 2 +: Iron now has an electron configuration of [Ar]3d6 [ A r] 3 d 6 after losing two electrons from its 4s orbital. The iron ion acquires a 2+ charge as a result. An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully .
To understand this conceptually, the superscripts are an electron. Boron's atomic number of 5 indicates that it has 5 electrons, and all the superscripts added up are equal to 5. The electron configuration is telling us that 2 electrons occupy the 1s orbital, 2 electrons occupy the 2s orbital, and one electron occupies the 2p orbital.Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. . 56 Fe Electron configuration [Ar] 3d 6 4s 2 CAS number: 7439-89-6 ChemSpider ID: The Electronic Configuration of Iron. A chemical element with an atomic number 26 and a symbol Fe represents it, Iron is a very common element that is found. Unlike other elements, iron exists at the oxidation states of -2 to +6. Elementary iron occurs in a low-oxygen environment in spite of being reactive to water and oxygen.The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon . Iron(II) loses two electrons and, since it is a transition metal, they are removed from the 4s orbital Fe 2+: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 = 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. (e). Sm: 1s 2 2s 2 2p 6 .

Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure.The chemical symbol for Iron is Fe. Electron Configuration and Oxidation States of Iron. Electron configuration of Iron is [Ar] 3d6 4s2. Possible oxidation states are +2,3. Electron Configuration. The periodic .
kantutan pronunciation with meanings, synonyms, antonyms, translations, sentences and more . with meanings, synonyms, sentence usages, translations and much more. . Armenian azerbaijan Basque Bengali Bosnian Bulgarian Burmese Catalan Chinese Croatian Czech Danish Dutch English Esperanto Estonian Filipino Finnish .
electron configuration of fe|electron configuration periodic table